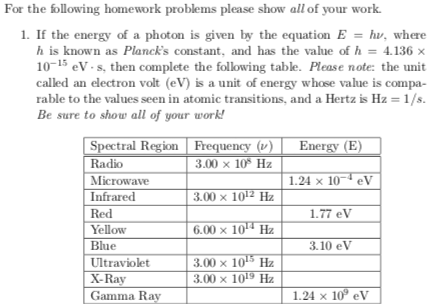
E Hv Problems
Here is another simple division problem.
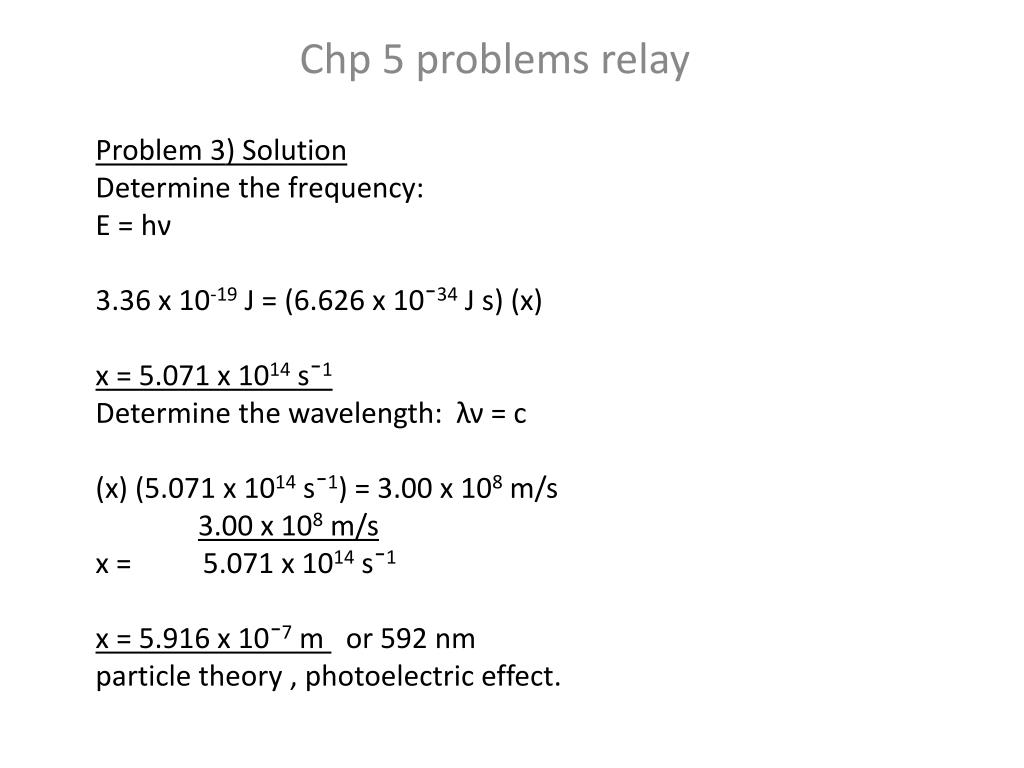
E hv problems. What is the energy of exactly one photon of this microwave radiation. 2 determine the frequency of the photon. This type of problem is good practice at rearranging equations using correct units and tracking significant figures. In 1900 max plank found a theoretical formula that exactly describes the intensity of light of various frequencies emitted by a hot solid at different temperature according to plan the atoms of the solid oscillate or vibrate with a definite frquency depending on the solid but in order to reproduce the results of experiments on he found it necessary to accept a strange idea a vibrating atom.
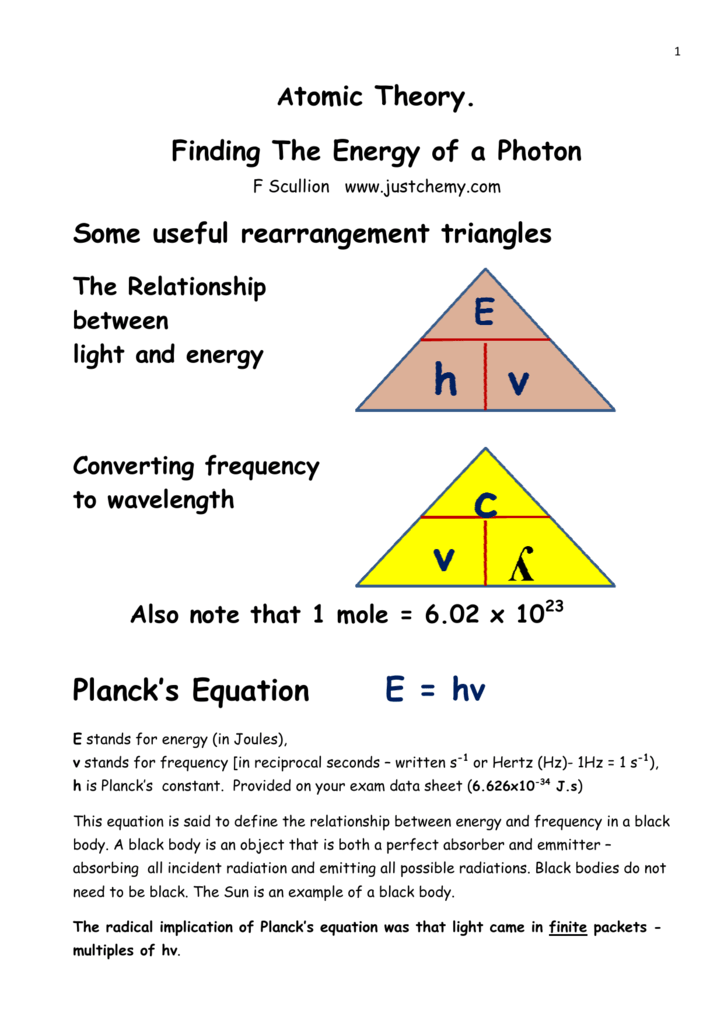
Is the frequency of the light in s 1 or waves s also called hertz hz. Energy plank s constant frequency e hv v e h v 1 4 10 21 j 6 626 10 34 js v 2 1 10 12 1 s for the love of physics walter lewin may 16 2011 duration. E hv is used to calculate the energy of electromagnetic radiation and is known as the planck einstein relation. This example problem demonstrates how to find the energy of a photon from its wavelength to do this you need to use the wave equation to relate wavelength to frequency and planck s equation to find the energy.
Calculate the wavelength and frequency of a photon having the energy of 8 93 x 10 10 j mol. E is the energy of electromagnetic radiation h is the planck constant and v is the frequency of electromagnetic radiation. Is the wavelength there are 1 x109 nanometers in one meter. 590 khz 590 10 3 hz 3 x 10 8 m s 590 x 10 3 hz 500m.
E hν 1 4829 x 10 13 j 6 626 x 10 34 j s ν ν 1 4829 x 10 13 j 6 626 x 10 34 j s. Is the speed of light. E is the energy of the light in joules j h. See formula is like dis.
Here we need to use two equations. C 3 00 x 108 meters sec. Is a constant which is 6 626 x 10 34 j s and. Microwave ovens emit microwave energy with a wavelength of 12 9 cm.
E hv c λv. Example of the use of e hv duration. Frequency of radiation f c l the place c is velocity of sunshine in vaccum ie 3 10 8 m sec and l is wavelength of the radiation so via putting wavelength 850 nm 850 10 9 m u gets frequency of given wavelength 3 529 10 14 and unit is sec a million wish u get d maximum suitable answer.