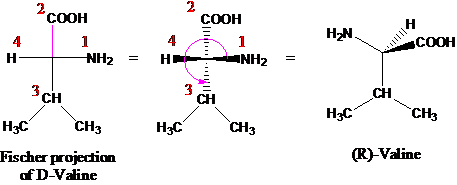
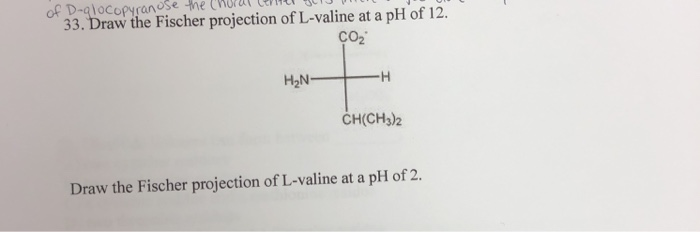D Valine Fischer Projection
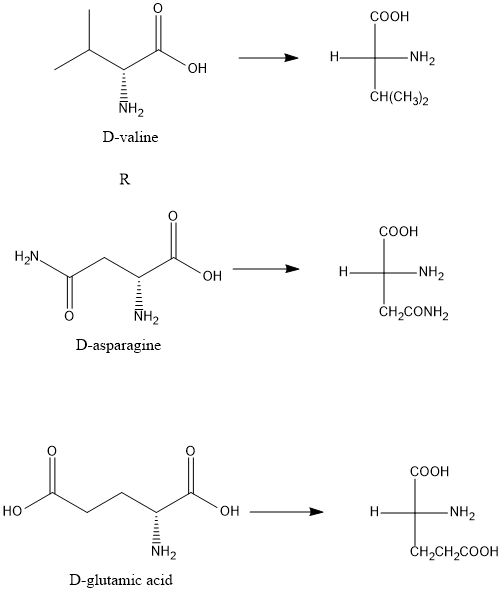
The antibiotic actinomycin d for example contains a unit of d valine and the antibiotic bacitracin a contains units of d asparagine and d glutamic acid.
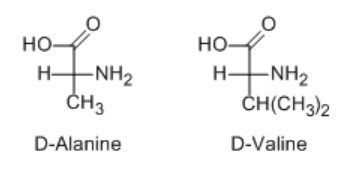
D valine fischer projection. This program does not recognized fischer projections. For tryptophan both ways of drawing the alternate single and double bonds in the benzene ring are allowed. Recall that fischer projections are typically drawn with the longest chain oriented vertically and with the more highly oxidised c at the top. Draw fischer projections for d alanine and d valine.
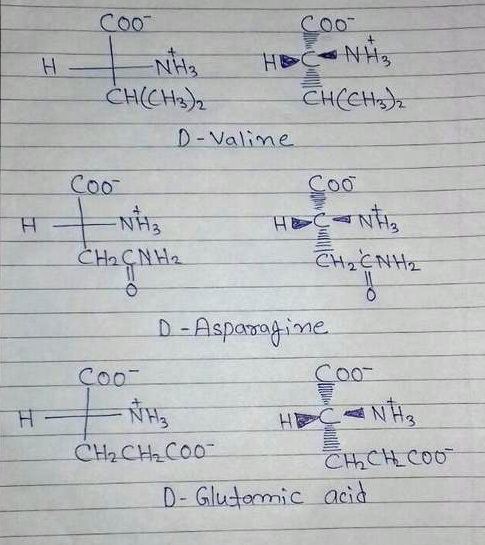
Draw fischer projections and three dimensional representations for these three d amino acids. R group is placed at the bottom of the. 4 2 fischer projections and ball and stick models of two rotameric minima for 1 123. In fischer projection formulas the terminal carbon that is most highly oxidized is placed at the top of the formula an aldehyde or.
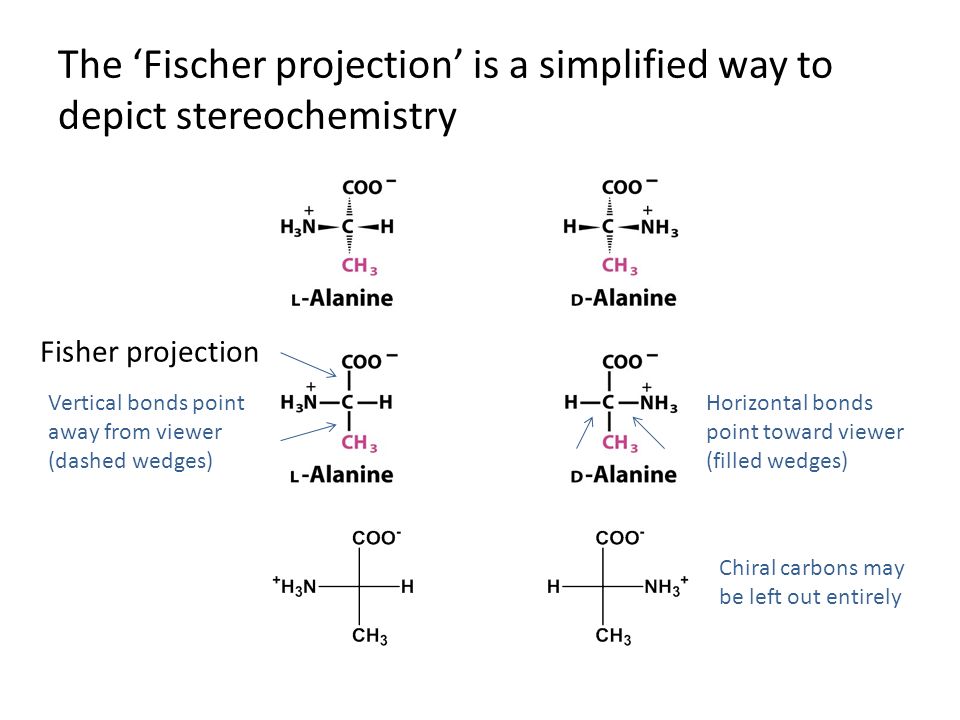
Cooh group is placed at the top of the vertical line of the fischer projection. The main carbon chain is drawn as a vertical line and bonds to all substituents are drawn as horizontal lines all vertical lines represent bonds behind the plane of the page all horizontal lines represent bonds in front of. Draw fischer projection formulas for the following amino acids. The rules for writing a fischer projection formula for amino acid are given below.
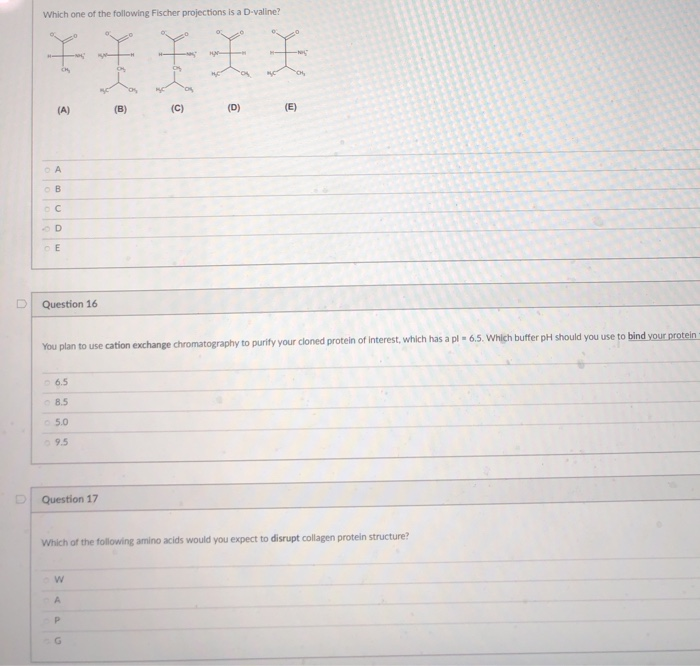
For the 20 α amino acids that occur naturally in proteins if we focus on. нс che нс ch hc ch a b c d e oa b c o d. Nh hn nh3 hn h nh ch ch2 нс сн. Which one of the following fischer projections is a d valine.
The l and d forms of alanine are shown in their standard fischer projections with the carbon chain written vertically. Acv d l a aminoadipoyl l cysteinyl d valine ao atomic orbital bl butyrolactone cardo carbazole 1 9a dioxygenase casscf complete active space self consistent eld cl caprolactone. Fischer projections are commonly used to represent amino acids. Fischer projections this convention proposed by emil fischer in 1891 attempts to depict molecular structure in a two dimensional framework of vertical and horizontal bonds.
Cooh h nh 2 ch ch 3 2 d valine cooh nh 2 h ch ch 3 2 cooh nh 2. The intersection of the horizontal and the vertical line represents the chiral centre. Amino acids really exist as alpha ammonium carboxylates because amino group are basic and carboxylic acids are acidic and side groups may also be protonated or deprotonated but this program does not assume that. Configuration at each chirality center.